2017年09月07日
Hydrogen Rich water helps in the treatment of Parkinson's Disease in Rats
Hydrogen Rich water helps in the treatment of Parkinson's Disease in Rats
https://youtu.be/5pwz1SqVzPw
Published on Sep 20, 2016
Category People & Blogs
License Standard YouTube License
~~~~~~~~~~~~~~~~~~~~~~~~~~~~
☆NOTE☆
"Parkinson's disease" :Wikipedia
https://en.wikipedia.org/wiki/Parkinson%27s_disease
"パーキンソン病" :Wikipedia
https://ja.wikipedia.org/wiki/%E3%83%91%E3%83%BC%E3%82%AD%E3%83%B3%E3%82%BD%E3%83%B3%E7%97%85
☆ Reference Link☆:
Hydrogen Rich water effect to Parkinson's disease
1. “Oral ‘hydrogen water’ induces neuroprotective ghrelin secretion in mice”
Akio Matsumoto, Megumi Yamafuji, Tomoko Tachibana, Yusaku Nakabeppu, Mami Noda & Haruaki Nakaya
https://www.nature.com/articles/srep03273
Abstract
The therapeutic potential of molecular hydrogen (H2) is emerging in a number of human diseases and in their animal models, including in particular Parkinson's disease (PD). H2 supplementation of drinking water has been shown to exert disease-modifying effects in PD patients and neuroprotective effects in experimental PD model mice. However, H2 supplementation does not result in detectable changes in striatal H2 levels, indicating an indirect effect. Here we show that H2 supplementation increases gastric expression of mRNA encoding ghrelin, a growth hormone secretagogue, and ghrelin secretion, which are antagonized by the β1-adrenoceptor blocker, atenolol. Strikingly, the neuroprotective effect of H2 water was abolished by either administration of the ghrelin receptor-antagonist, D-Lys3 GHRP-6, or atenolol. Thus, the neuroprotective effect of H2 in PD is mediated by enhanced production of ghrelin. Our findings point to potential, novel strategies for ameliorating pathophysiology in which a protective effect of H2 supplementation has been demonstrated.
Introduction
Therapeutic applications of molecular hydrogen (H2) have been reported in a variety of human diseases and their animal models1, including ischemia-reperfusion injury2,3,4, metabolic syndrome5, diabetes mellitus type 26, organ transplantation7,8,9, reduction of adverse effects of anti-tumor drug therapy10,11 and radiation therapy12,13. Although the mechanism of action of H2 has not been clearly demonstrated, it is assumed that its anti-oxidative properties, particularly against hydroxyl radical (・OH) and peroxinitrite (ONOO−), are likely to underlie therapeutic efficacy2. Unlike other medical-gas therapy, H2 can be applied in air for inhalation or in solution for drinking, intravenous injection or dialysis. Whereas intravenous injection or dialysis delivers H2 directly into the blood stream, oral hydrogen-supplemented water (hydrogen water, H2-water) must be absorbed into the circulation resulting in limited H2 concentrations in the blood and in target organs7,14.
Parkinson's disease (PD) has been a major focus in the field of oxidative stress and disease, because it is thought that degeneration of dopaminergic neurons can be triggered and aggravated by the accumulation of oxidative damage. However, although antioxidant therapies have been assessed in PD patients, clinical efficacy has not been established15,16. In contrast, a pilot study of hydrogen water therapy in PD patients has shown promising results17, and it has been reported that hydrogen water exhibits neuroprotective effects14 in the murine MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine)-induced PD model18. H2 levels were below measurable thresholds in the substantia nigra in PD model mice14, and hydrogen water, but not continuous inhalation of 2% H2, prevented the development of PD in a rat model19. These findings suggest that the therapeutic effects of hydrogen water may not require its anti-oxidant activity in the brain, and further that its efficacy requires processing that is consequent upon oral administration.
The purpose of the present study was to employ PD model mice to elucidate the underlying mechanism of the neuroprotective effects of oral H2-water. In particular, we hypothesized that oral H2 induces a messenger molecule, which travels to the brain and exerts neuroprotective activity.
Results
Oral hydrogen water induces ghrelin gene expression in the stomach
The stomach functions as an endocrine organ that secretes various peptide hormones with a broad range of physiological effects. We first focused on the stomach to investigate possible effects of oral H2-water at the level of gene induction. In a previous study14, it was reported that drinking H2-water for a period of 7 days prior to MPTP injection protected against MPTP toxicity. We administered oral H2-water for 4 consecutive days and analyzed expression in stomach tissue of gastrin, somatostatin, and ghrelin by real-time PCR method. Levels of ghrelin mRNA increased 1.9-fold in H2-water-treated versus control mice (Figure 1), whereas no effects of hydrogen water were detected on expression of the somatostatin gene (expressed only at trace levels) or the gastrin gene (expression of which was highly variable from individual to individual) (not shown).
Figure 1: Oral H2 water increases ghrelin gene expression in the mouse stomach.
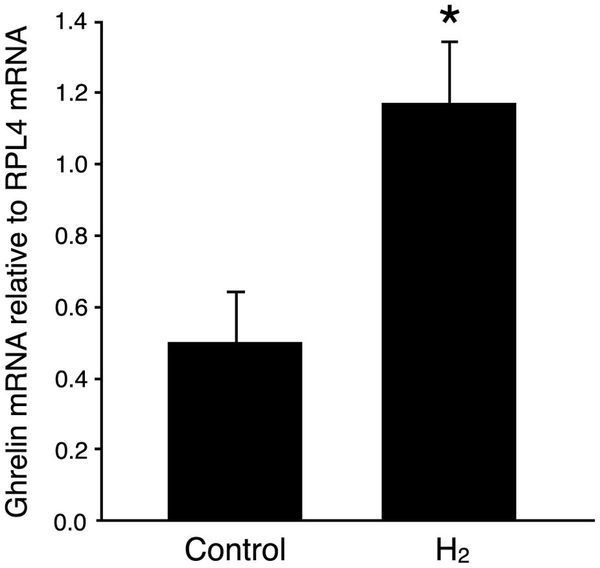
Control water, or H2 water (0.8ml/mouse) made with a stick of magnesium (about 0.04mM H2; see Methods), was administered to mice (41–48 weeks of age; n = 4 per group) once a day for 4 days. Three hr after the final ingestion, the stomach was removed and prepared for qPCR. Data were normalized with respect to expression of RPL4 mRNA and are represented as mean ± SEM.
To examine the time-course of ghrelin induction by hydrogen water, mice were administered hydrogen water or control water once a day for 0 (control) 1, 2 or 4 days and ghrelin levels were measured by ELISA in plasma derived from blood obtained 4–5hours after the final administration of H2- or control water. Only mice that received H2 water for 4-day exhibited a significant increase in plasma ghrelin level, although mice administered hydrogen water for 2-day showed a non-significant increase (Figure 2).
Figure 2: Plasma ghrelin levels following administration of oral H2 water.
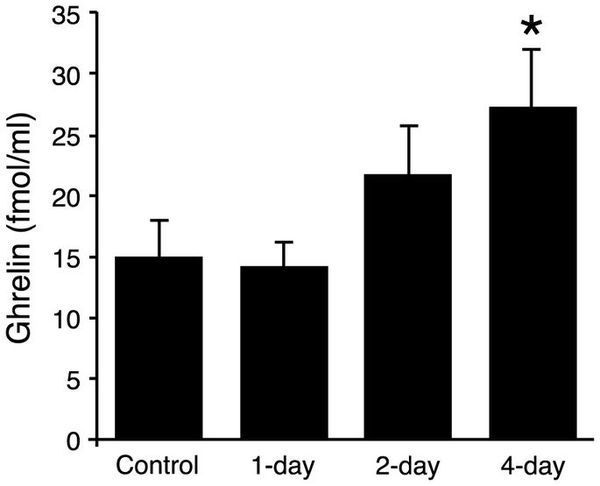
Mice (16–21 weeks, average 18.5 weeks, n = 5–10) received H2 water (or control water) as in Fig. 1 for 1, 2 or 4d. Blood was collected on the final day of the experiment, 4hr after the last ingestion, and plasma levels of ghrelin were quantified by ELISA. Panel shows data from male mice. The ghrelin level was significantly higher only in the group of mice drinking H2 water for 4d. Data are represented as mean ± SEM.
Mice (16–21 weeks, average 18.5 weeks, n = 5–10) received H2 water (or control water) as in Fig. 1 for 1, 2 or 4d. Blood was collected on the final day of the experiment, 4hr after the last ingestion, and plasma levels of ghrelin were quantified by ELISA. Panel shows data from male mice. The ghrelin level was significantly higher only in the group of mice drinking H2 water for 4d. Data are represented as mean ± SEM.
β1-adrenergic receptor signaling mediates enhancement of ghrelin secretion by oral hydrogen water
It has been shown that gastric secretion of ghrelin is regulated by local environmental cues including blood glucose, estrogen, insulin and catecholamines20,21,22. In particular, it has been reported that β1-adrenergic receptor stimulation increases ghrelin secretion in vitro and in vivo22,23. We verified the expression of β1- and β2- adrenergic receptors in stomach tissue samples by real-time PCR method, and determined that there were no significant changes in the levels of expression after administration of oral H2-water for 4 days (data not shown). The increase in plasma ghrelin levels by oral hydrogen water was eliminated by administration of the β1-adrenergic receptor-specific blocker, atenolol (10mg/kg i.p.) injected 30min prior to H2 water administration on each of four days (Figure 3). Thus, activation of β1-adrenergic receptors is required for hydrogen water-induced enhancement of circulating ghrelin.
Figure 3: H2 water increases plasma ghrelin levels in a β1-adrenergic receptor-dependent manner.
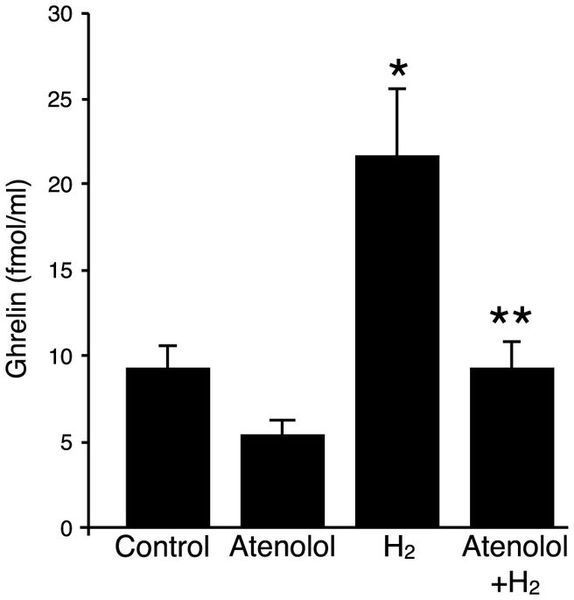
Plasma ghrelin levels were measured on the last day of experiment. Each group of mice (11–13 weeks of age; n = 4–5 for each group) was administered control water or H2 water (made with an open-air water electrolysis system) once a day for 4d with or without i.p. injection of atenolol (10mg/kg) prior to water ingestion. The H2 water group showed a significant increase in plasma ghrelin level compared to the control group (* p = 0.038), which was abrogated by pretreatment with atenolol (** p = 0.039). Datafare represented as mean ± SEM.
Blockades of ghrelin action abrogates the protective effect of H2 water in PD model mice
In a previous report, oral H2-water exhibited significant protective effects in MPTP-induced PD model mice against the loss of dopaminergic neurons from the substantia nigra14. To test directly the role of ghrelin, either the growth hormone secretagogue receptor antagonist, D-Lys3 GHRP-6 (100nmol/day i.p.), or β1-adrenoceptor blocker, atenolol (10mg/kg i.p.), was administered along with control or hydrogen water for 7 days prior to administration of MPTP. Loss of dopaminergic neurons from the substantia nigra was evaluated seven days following administration of MPTP. As shown in Figure 4, systemic administration of MPTP caused a significant loss of dopaminergic neurons from the pars compacta of the substantia nigra (SNpc) as assessed by immunohistochemical detection of tyrosine hydroxylase (TH) (a), and further confirmed by immunological detection of TH protein in the substantia nigra with actin as control (b), and stereological analysis (c). Whereas administration of H2 water alone in control mice had no effect, the loss of dopaminergic neurons in MPTP-treated mice was significantly decreased by administration of hydrogen water, as previously reported14. Strikingly, although administration of D-Lys3 GHRP-6 or atenolol in control mice had no effect alone and D-Lys3 GHRP-6 or atenolol did not affect MPTP-induced loss of dopaminergic neurons, the protective effects of hydrogen water were eliminated by either one of D-Lys3 GHRP-6 or atenolol. Thus, induction of gastric ghrelin production and subsequent activation of ghrelin-initiated signal transduction underlies the protective effects of hydrogen water in the MPTP model of PD.
Figure 4: Inhibition of ghrelin secretion or the ghrelin receptor antagonist cancels the neuroprotective effect of oral H2 water in MPTP-induced Parkinson's disease model mice (male, 8–12 weeks, n = 2–5 for each group).
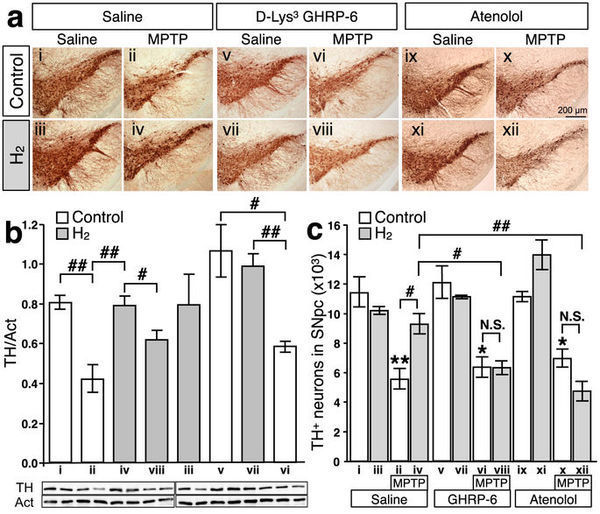
Saturated H2 water was made daily as described in materials and methods. (a): Tyrosine hydroxylase (TH) staining was performed in substantia nigra pars compacta from saline-injected (i–iv), D-Lys3 GHRP-6-injected (v–viii), and atenolol-injected (ix–xii) mice, supplied with either normal tap water (control) or H2 water for 7 days. MPTP was injected (i.p.) after 7 days of D-Lys3 GHRP-6, atenolol, or saline-injection (ii, iv, vi, viii, x, and xii). (b): Summary of the immunoblotting analysis of TH protein in the substantia nigra tissue (n = 3–6 for each group) and the representative blot images were shown underneath (two samples per group). The TH band intensity was normalized to the band of actin on the same sample. (## p = 0.007, i vs. ii; 0.010, ii vs. iv; 0.004, vi vs. vii; # p = 0.043, iv vs. viii; 0.023, v vs. vi). Data in figures are represented as mean ± SEM. (c): Summary of the stereological analysis of nigral dopaminergic neurons. Although MPTP injections caused significant loss of TH-positive neurons (ii) (** p = 0.0001, i vs. ii), drinking H2 water for 7 days prior to MPTP-treatment significantly attenuates the loss of TH-positive cells (iv) (# p = 0.008, ii vs. iv). D-Lys3 GHRP-6, growth hormone secretagogue receptor antagonist, or β1-adrenoceptor blocker, atenolol, canceled the preservation effect of oral H2 water (viii and xii) (# p = 0.008; ii vs. iv; p = 0.003, iv vs. viii; p = 0.0003, iv vs. xii). Data in figures are represented as mean ± SEM. The statistical significance of data was assessed by one-way ANOVA followed by Benferroni test.
Discussion
Our findings demonstrate that the neuroprotective effects of oral hydrogen water, which produces negligible levels of H2 in the brain, result from gastric induction of the neuroprotective peptide hormone ghrelin and the subsequent activation of ghrelin receptors. In addition, we have shown an obligate role for β1-adrenergic receptors in hydrogen water-induced ghrelin up-regulation in plasma, consistent with previous reports that adrenergic stimulation regulates ghrelin release in vitro and in vivo22,24,25.
The neuroprotective effects of ghrelin in PD are well-established26, and it has been demonstrated that the receptor for ghrelin, the growth hormone secretagogue receptor (GHSR), is highly expressed by dopaminergic neurons of the substantia nigra27. It has been suggested that ghrelin protects nigrostriatal dopamine neurons via an uncoupling protein 2 (UCP2)-dependent mitochondrial mechanism28,29. However, we found that the expression of neither UCP2 mRNA nor protein was significantly upregulated by administration of H2 water drinking for 7 days (data not shown). This finding suggests an alternative signaling mechanism downstream of GHSR activation, perhaps involving PI3K/Akt30.
It was reported that administration of saturated hydrogen water (approx. 0.8mM) led to symptomatic improvement in PD patients17. Administration of hydrogen water at about 0.05% saturation successfully maintained dopaminergic neurons in MPTP-induced PD model mice14. We employed three different methods to prepare hydrogen water (see Methods), which resulted in H2 concentration of 0.04–0.8mM, and we observed that the effects of hydrogen water on ghrelin induction and protection of dopamine neurons were dose-independent over this range. Thus, small amounts of oral H2 are sufficient for gastric induction of ghrelin and subsequent neuroprotection, in the absence of detectable H2 in the brain. Interestingly, gut microbes can produce molecular hydrogen constitutively and lactulose, a synthetic disaccharide, is an effective substrate to enhance bacterial H2 production in the colon31. However, ingestion of lactulose had no significant effect on dopaminergic neuron survival in 6-OHDA-induced PD model rats (although alveolar H2 concentrations were elevated)19. These results emphasize the importance of gastric ghrelin induction in the neuroprotective action of H2. Oral H2-water is being explored as a therapeutic for PD as well as a variety of other human pathophysiological conditions17,32,33 under the generally held assumption that the mechanism of action of supplemental H2 is likely to reflect an antioxidative role. Our findings that oral H2 water exerts a neuroprotective effect through activation of an endogenous, gastric ghrelin system that is tightly coupled to β-adrenergic receptor signaling suggests the possibility of novel applications of H2 therapy for various diseases.
The rest is omitted.
〜〜〜〜〜〜〜〜〜〜
〜〜〜〜〜〜〜〜〜〜
~~~~~~~~~~~~~~~~~~~~~~~~~~~~
☆ Reference Link☆:
Hydrogen Rich water effect to Parkinson's disease
2. Blog:
“We love to write and share information Hydrogen Rich Water, Water Filters, Water, and many other health-related issues”
http://www.californiahydrogenwater.com/blog/new-study-on-effects-of-h2-on-parkinsons-disease
New Study on Effects of H2 on Parkinson's Disease
Here are the details of a new H2 study being conducted on people with Parkinson’s Disease. 178 individuals (89men/89 women average age 64.2 yrs.) in 14 different hospitals will be tested over the course of 72 weeks. Half will be given 1 liter of H2 water per day, half will drink regular water. Most of the patients are on levodopa which is used sometimes alone and sometimes with other drugs to hep with the symptoms of PD.
Previous studies have shown H2 to be of benefit with PD. This study is to confirm those findings with a “randomized double-blind placebo-controlled multi-center trial”.
Click here to read more
https://www.ncbi.nlm.nih.gov/pubmed/27176725
===================================================
===================================================
===================================================
===================================================
【このカテゴリーの最新記事】
-
no image
この記事へのコメント
コメントを書く


